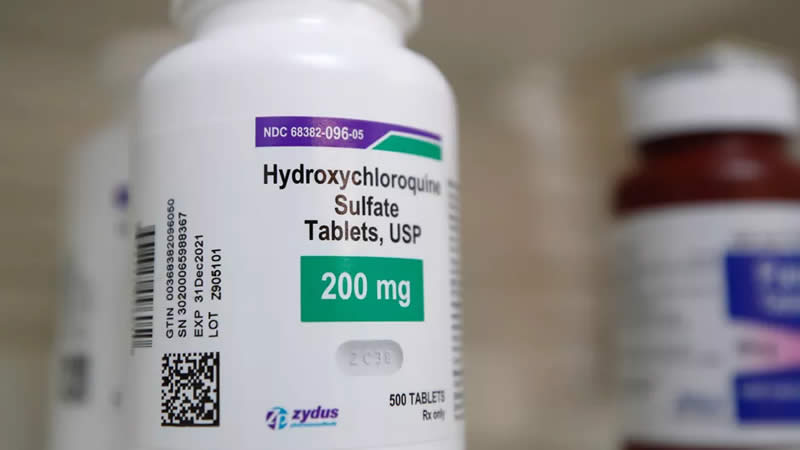
The World Health Organization (WHO) said Monday that due to safety concerns, it is temporarily halting a clinical trial of the anti-malaria drug hydroxychloroquine for treating coronavirus patients.
The announcement follows the publication Friday by the Lancet medical journal of an observational study on hydroxychloroquine and chloroquine and its effects on hospitalized COVID-19 patients.
“The Executive Group has implemented a temporary pause of the hydroxychloroquine arm within the Solidarity Trial while the safety data is reviewed by the Data Safety Monitoring Board,” said WHO Director-General Tedros Adhanom Ghebreyesus.
He said that more than two months ago, the WHO initiated its “Solidarity Trial” to evaluate the safety and efficacy of four drugs and drug combinations against COVID-19.
On April 19, US President Donald Trump said he had been taking hydroxychloroquine, which he has repeatedly touted for the treatment of coronavirus patients.
According to the WHO, over 400 hospitals in 35 countries are actively recruiting patients and nearly 3,500 patients have been enrolled from 17 countries under the Solidarity Trial.
Tedros added that the safety concern over the drug related only to the use of HCQ and chloroquine in COVID-19, and “these drugs are accepted as generally safe for use in patients with autoimmune diseases or malaria”.
“WHO will provide further updates as we know more,” he added.
Meanwhile, the United States’ Food and Drug Administration has cautioned against the use of Hydroxychloroquine on COVID-19 patients even as President Donald Trump, who has touted it as a “game changer,” advocated for an additional review.
Hydroxychloroquine, first approved in 1955, provided no benefit and potentially higher risk of death for patients at US veterans hospitals, according to an analysis that was submitted for an expert review in April.
The FDA’s announcement came a day after the European Union’s drug regulator warned of side effects of the drug, urging medical professionals to closely monitor patients on the medicine.The FDA has allowed healthcare providers to use the drugs for COVID-19 through its emergency use authorization, but has not approved them to treat the disease.
The US acquired 29 million doses of Hydroxychloroquine from India, and at the request of President Donald Trump, it cleared the export of 35.82 lakh tablets of the drug to the US along with nine metric tons of active pharmaceutical ingredient or API required in the manufacturing of the drug.
Meanwhile, India has decided to expand the use of Hydroxychloroquine as prophylactic for asymptomatic healthcare workers as well those engaged in fighting COVID-19 coronavirus.
A revised government advisory last week recommended use of hydroxychloroquine as a preventive medication for asymptomatic healthcare workers working in non-COVID-19 hospitals, frontline staff on surveillance duty in containment zones and paramilitary/police personnel involved in coronavirus infection related activities.